Aseptic Manufacturing
CreoSalus, Inc. operates a cGMP facility designed and approved for aseptically manufactured or terminally sterilized parenteral and veterinary injection pharmaceuticals. Our 20+ years of pharmaceutical manufacturing experienced team will provide development and engineering support from the start of the manufacturing process, including special equipment identification and validation, through product formulation, fill and finish for clinical supplies to post approval commercial manufacturing.
Aseptic filling of sterile drugs is one of the most critical and challenging processes in pharmaceutical manufacturing. Let our experienced team take care of all your parenteral and veterinary drug aseptic filling needs. CreoSalus operates a cGMP facility designed and approved for aseptically manufactured or terminally sterilized parenteral and veterinary pharmaceuticals for clinical trials through commercial filings.
We specialize in analytical method and manufacturing process research and development with fill batch sizes capacity of 15,000 units. We welcome small scale projects that may not meet the minimum vial requirement of other larger aseptic fill companies. CreoSalus supports a variety of finished dosage forms and batch sizes ranging from engineering batches to small clinical trials through small scale commercial batches. Our experienced team will provide development and engineering support from the start of the development process, including special equipment identification and validation, through product formulation and finish with regulatory filing assistance and post approval commercial manufacturing.
OUR PROCESS
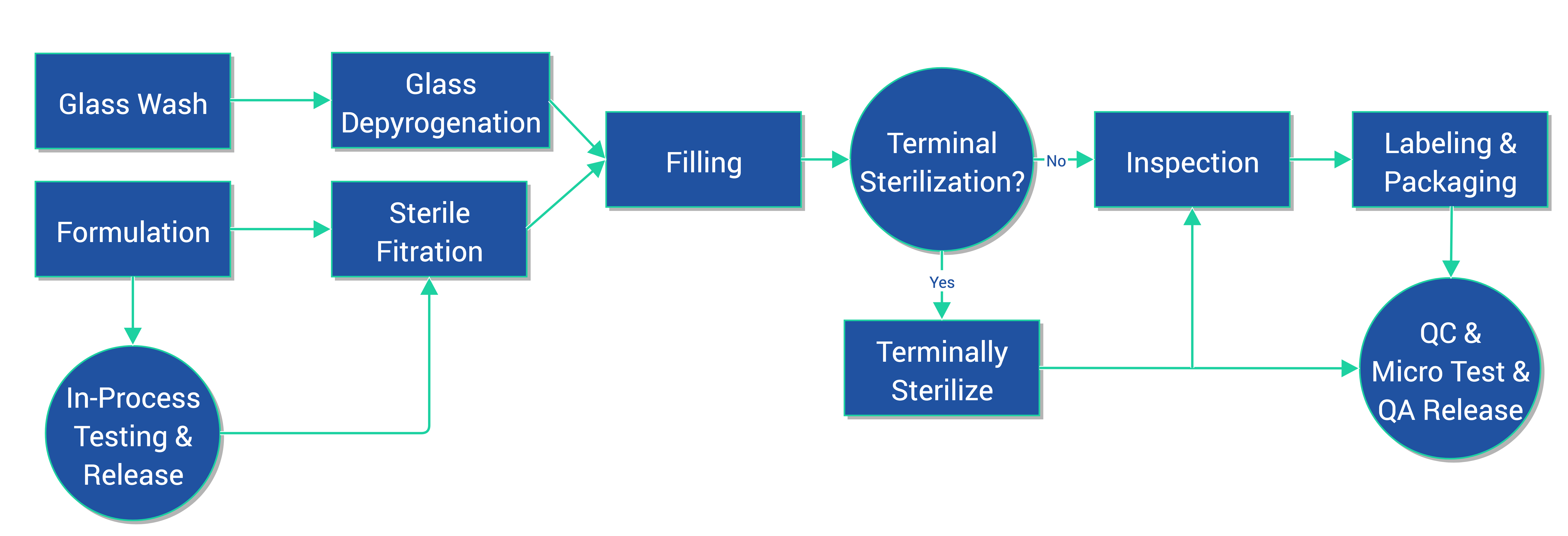
- Liquid and Suspension formulation processing equipment
- Metromatic Vial Washer
- Despatch Depyrogenation Oven
- Chase Logeman filling line in an ISO 5 enclosure for filling 2mL to 100mL vials
- Media Fills
- Sterilization cycle development, autoclave and terminal gamma irradiation
- Analytical Instrument: UPLC/MS, HPLC, KF, IR, GC, UV-VIS, Elemental Analysis, Particle Sizing
