cGMP CONTRACT MANUFACTURING
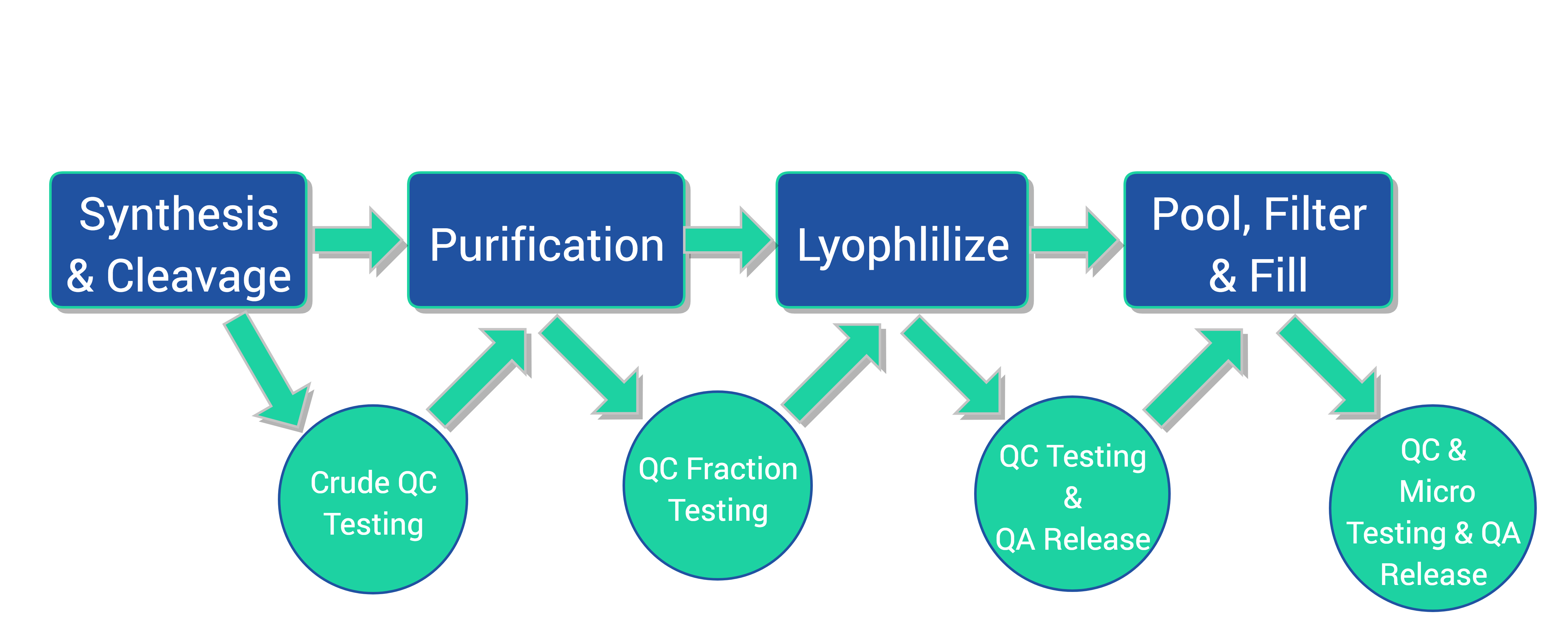
CreoSalus, Inc. has multiple high throughput cGMP peptide production lines and a fully equipped quality control lab capable of producing hundreds of API peptides monthly. The peptides can be packaged as a lyophilized powder or in cryovials through aseptic filling of the formulated peptides for individualized patient as well as bulk patient treatment. With years of experience, CreoSalus is a leading player in the high throughput cGMP personalized peptide production field, providing customers with high quality products, fast turnaround time and IND and INAD compliance.
Our Process
- Fully automated, in-house custom designed Synthesizers (6) – flexible capacity from low milligram to gram scale, producing up to 100 peptides simultaneously
- Prep-LC/MS Purification systems (8) – hundreds of gram loading capacity and mass-precision auto-collection
- Lyophilization (4) – 10L capacity each, suitable for peptide freeze drying
- ISO 5 Filling Chamber (2)
- Analytical Instrument: UPLC/MS, HPLC, KF, IR, GC, UV-VIS, Elemental Analysis